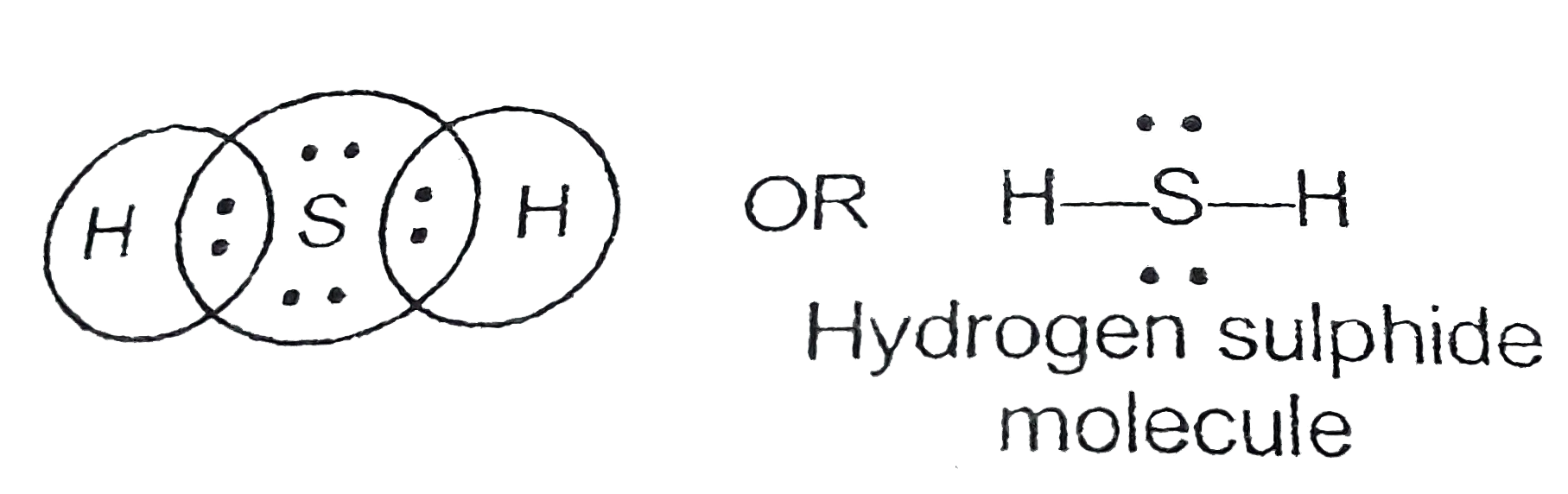
31+ H2S Lewis Structure Pictures Bepe Enthusiastic
Step 1: Figure out how many electrons the molecule must have, based on the number of valence electrons in each atom. When drawing the structure of an ion, be sure to add/subtract electrons to account for the charge. Step 2: Connect the atoms to each other with single bonds to form a "skeleton structure.".

H2, Hydrogen Lewis structure Chemistry worksheets, Lewis, Dots
Lewis structures, also known as Lewis-dot diagrams, show the bonding interactions between atoms in a molecule and lone pairs of electrons in the molecule.. To sketch the Lewis structure of H2, we must first determine the total number of valence electrons present in the H2 molecule. Hydrogen is a group 1 element on the periodic table.
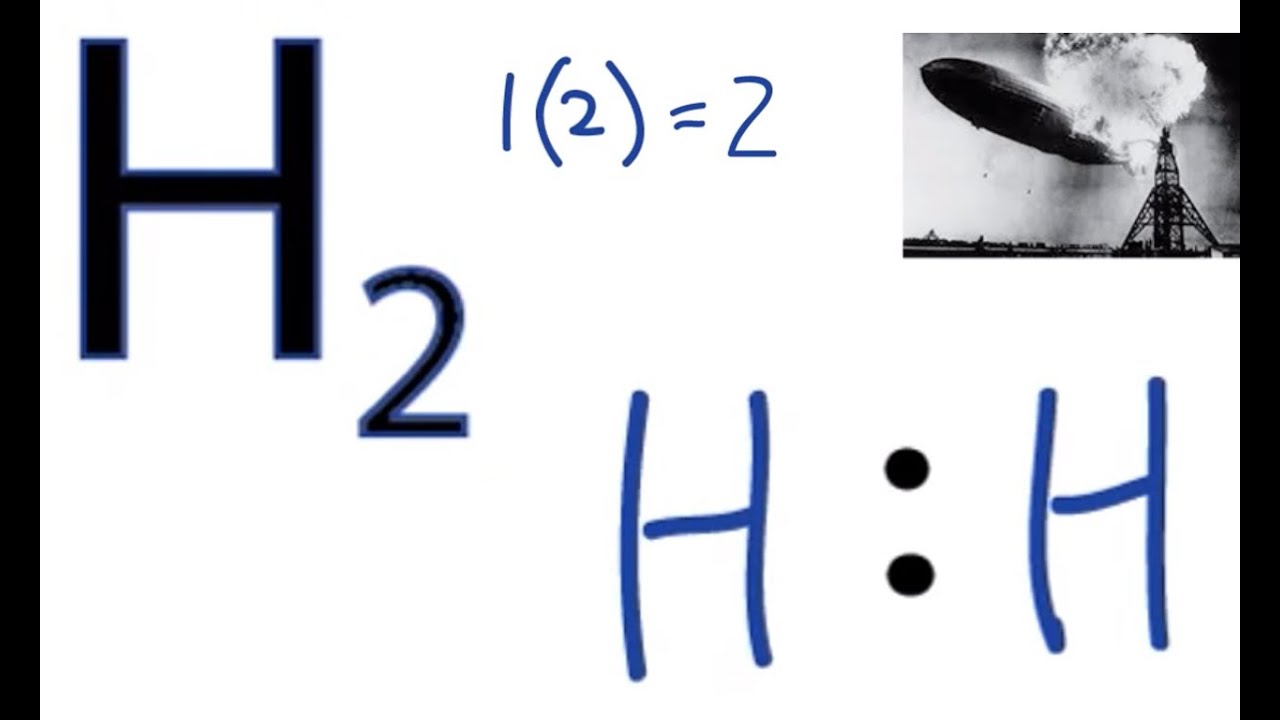
H2 Lewis Structure How to Draw the Dot Structure for H2 YouTube
This widget gets the Lewis structure of chemical compounds. Get the free "Lewis Structure Finder" widget for your website, blog, Wordpress, Blogger, or iGoogle. Find more Chemistry widgets in Wolfram|Alpha.

So far, we’ve used 8 of the H2S Lewis structure’s total 8 outermost
Also, the 2 valence electrons of H2 molecule (as calculated in step #1) are used in the above structure. And hence, the above lewis structure of H2 is the final stable structure only. The electron pair (:) in the lewis dot structure of H2 represents the single bond ( | ). So the above lewis dot structure of H2 can also be represented as shown.
Lewis Dot Diagram For H2 General Wiring Diagram
This type of Lewis dot structure is represented by an atomic symbol and a series of dots. See the following examples for how to draw Lewis dot structures for common atoms involved in covalent bonding. Example 1. Draw the Lewis Dot Structure for the Hydrogen atom. Since Hydrogen is in Group I it has one (1) valence electron in its shell.

H2 (hydrogen gas) Lewis dot structure and polarity YouTube
In the above structure, you can see that the central atom (right hydrogen) forms a duet. And the outside atom (left hydrogen) also forms a duet. Hence, the duet rule is satisfied. Therefore, this structure is the stable Lewis structure of H 2. Next: N 3 - Lewis structure. 📝 Your feedback matters. Visit our contact page.

draw the lewis dot structure of h2 Brainly.in
We can illustrate the formation of a water molecule from two hydrogen atoms and an oxygen atom using Lewis dot symbols: The structure on the right is the Lewis electron structure, or Lewis structure, for H 2 O. With two bonding pairs and two lone pairs, the oxygen atom has now completed its octet. Moreover, by sharing a bonding pair with oxygen.
.PNG)
Chemical Bonding revised Presentation Chemistry
Lesson 4: Dot structures and molecular geometry. Drawing dot structures. Drawing Lewis diagrams. Worked example: Lewis diagram of formaldehyde (CH₂O) Worked example: Lewis diagram of the cyanide ion (CN⁻) Worked example: Lewis diagram of xenon difluoride (XeF₂) Exceptions to the octet rule. Counting valence electrons.
Electron Dot Diagram For H2 Free Diagram For Student
A step-by-step explanation of how to draw the H2 Lewis Dot Structure (Hydrogen gas).For the H2 structure use the periodic table to find the total number of v.
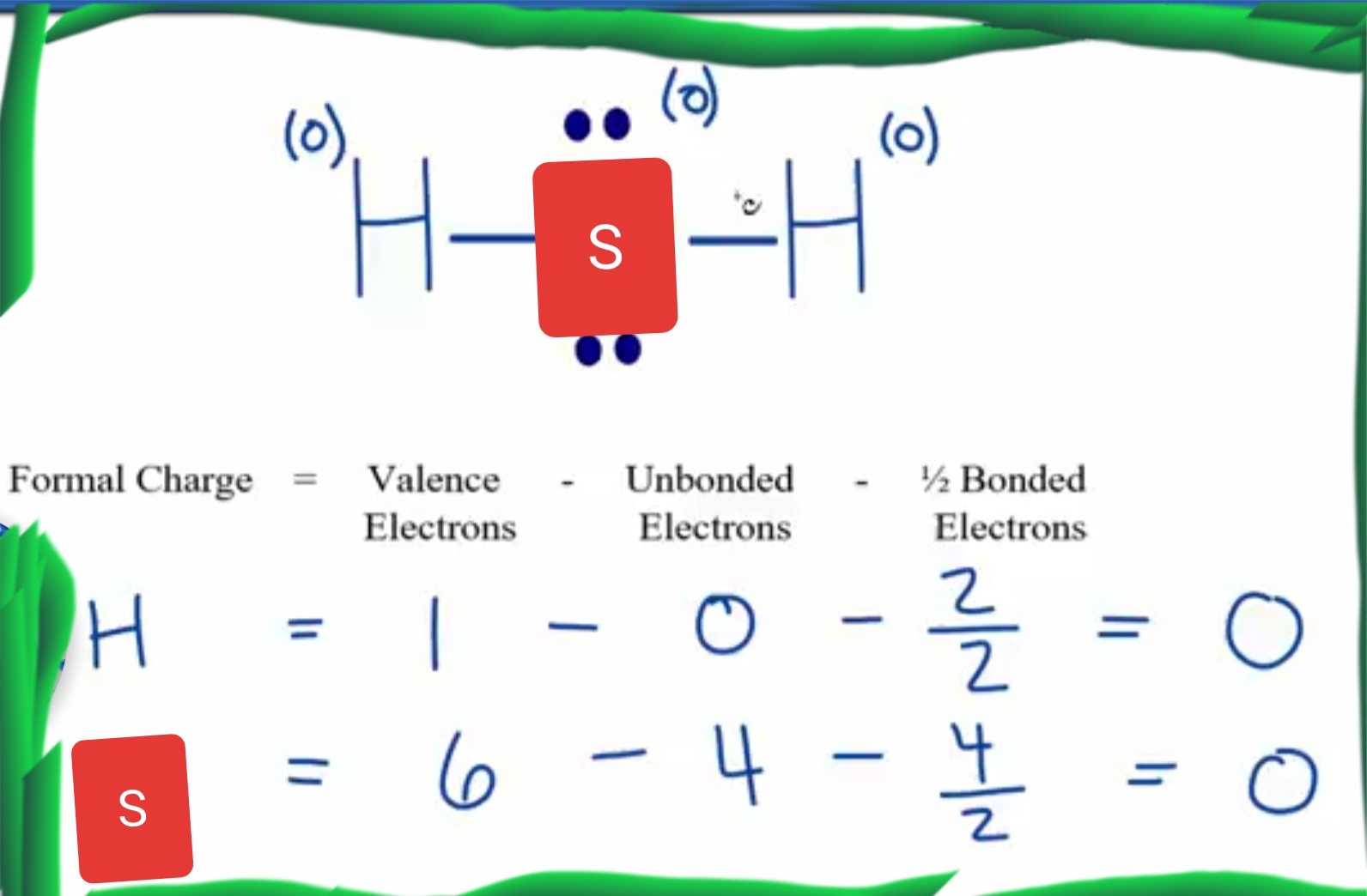
【4 Steps】H2S Lewis StructureLewis Structure for H2S (Dihydrogen
Follow these simple steps to draw Lewis dot structures: Draw the atoms on paper and put dots around them to represent valence electrons of the atom. Be sure to have the correct number of electrons. If the species is an ion, add or subtract electrons corresponding to the charge of the ion. Add an electron for every negative (-) charge, and.

【2 Steps】Lewis Dot Structure for Hydrogen(H,H2)Lewis Dot Structure of
A step-by-step explanation of how to draw the C2H2 Lewis Dot Structure (Ethyne or Acetylene).For the C2H2 structure use the periodic table to find the total.

1. Lewis Dot Structure of h2 How to Draw Lewis Structures Class 11
The electron dot structure of H 2: The total number of valence electrons present in H 2 = 2 . Here the central atom is the hydrogen itself. Each hydrogen has 1 valence electron, which will be used for the single bond formation between two hydrogen atoms, to fulfil their octet configuration. Hence, the electron dot structure of H 2 can be given as :
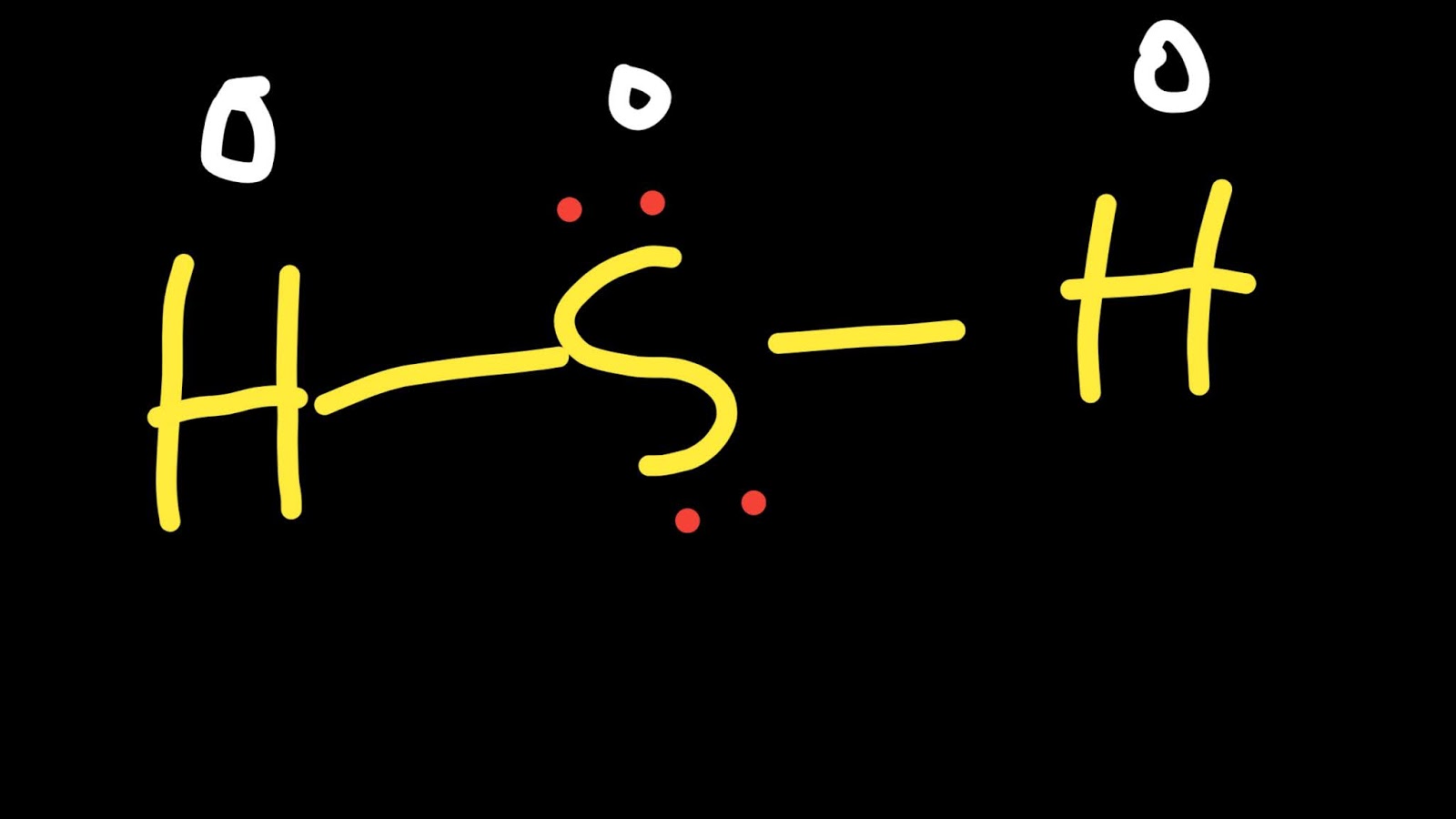
H2S Lewis Structure ,Valence Electrons ,Formal Charge,Polar or Nonpolar
A step-by-step explanation of how to draw the H2 Lewis Dot Structure (Diatomic Hydrogen).Note that Diatomic Hydrogen is often called Molecular Hydrogen or ju.

H2O Lewis Structure, Molecular Geometry, and Hybridization
Hey everyone, welcome to the Mentor Center! In today's video, I draw the Lewis dot structure of hydrogen gas (H2) and determine whether it is polar or nonpol.

Lewis Dot Diagram For H2 General Wiring Diagram
The H 2 Lewis structure is also one of the simpliest to draw. Hydrogen is in Group 1 and therefore has only one valence electron. Hydrogen atoms only need 2 valence electrons to have a full outer shell. Transcript: Hi, this is Dr. B. Let's do the Lewis structure for H2, Hydrogen gas. It's a quite explosive gas, so please don't fill your blimp.

How to Draw the Lewis Dot Structure for H2 Diatomic Hydrogen YouTube
The H2 molecule has a total 2 valence electrons and both these valence electrons are used in the above sketch of H2. In the above lewis dot structure of H2, you can also represent each bonding electron pair (:) as a single bond (|). By doing so, you will get the following lewis structure of H2. I hope you have completely understood all the.