Oxidation Number (State) Definition, Rules, How to Find, and Examples
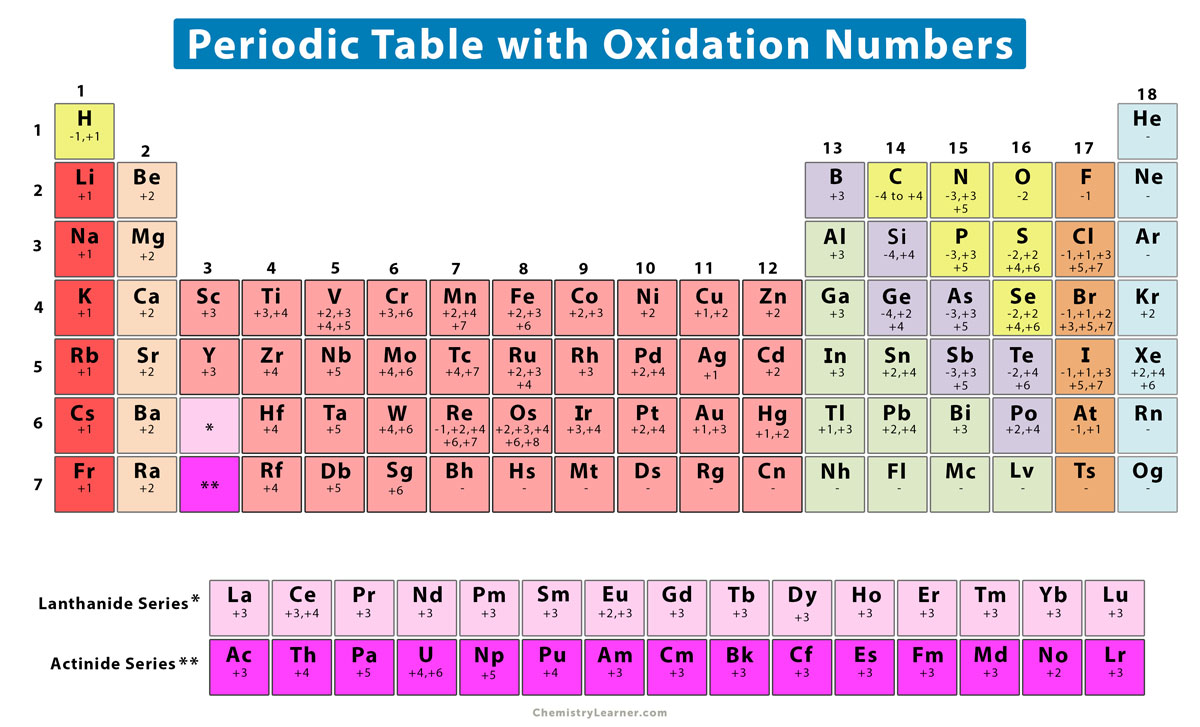
This printable periodic table contains the number, symbol, name, atomic mass and oxidation states of each element. The most common oxidation states are in bold text and predicted or unconfirmed states are in italics. This table is available for download as a PDF file and printed for offline use. For best printing, choose Landscape and 'Fit.
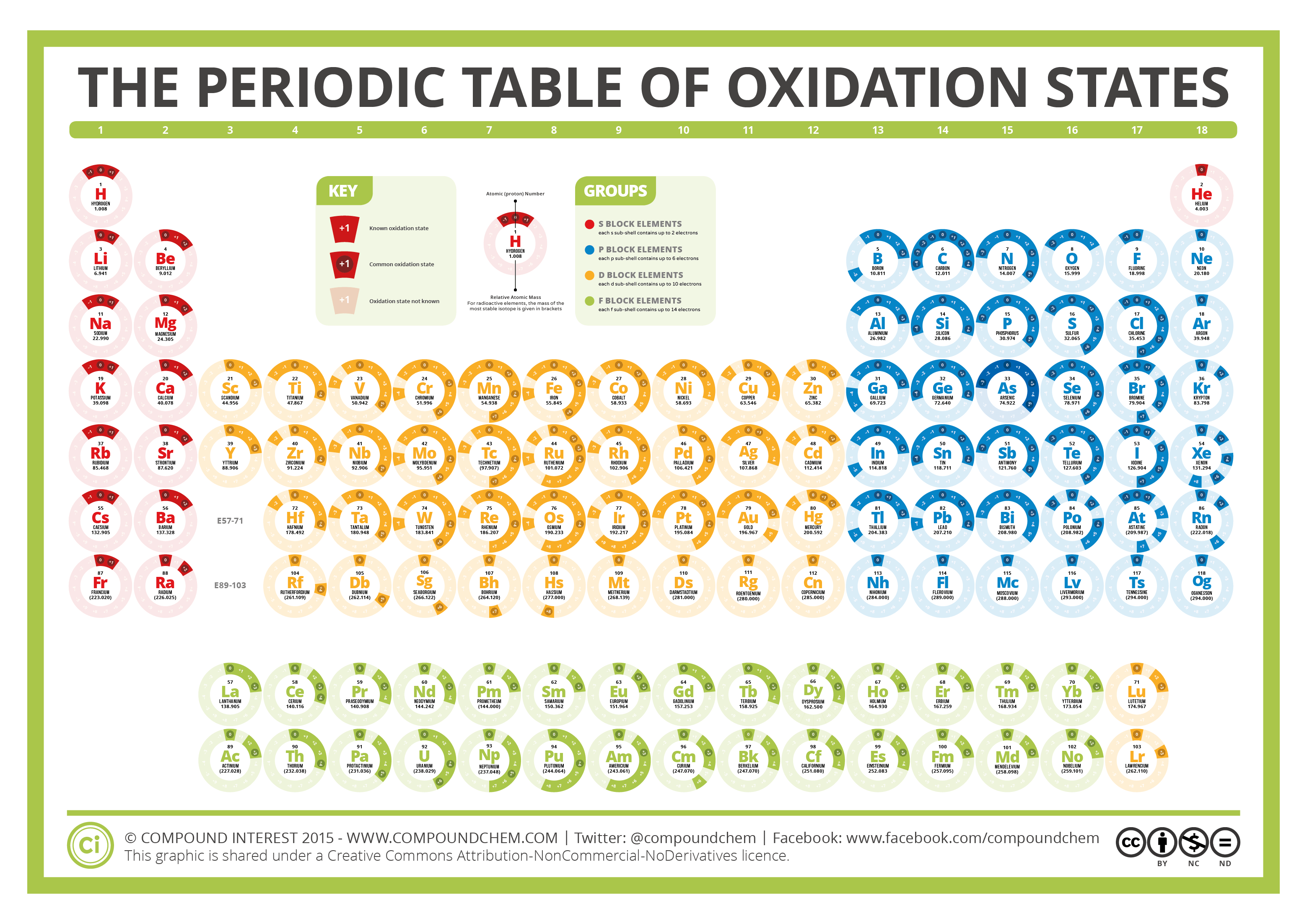
The Periodic Table of Oxidation States Compound Interest
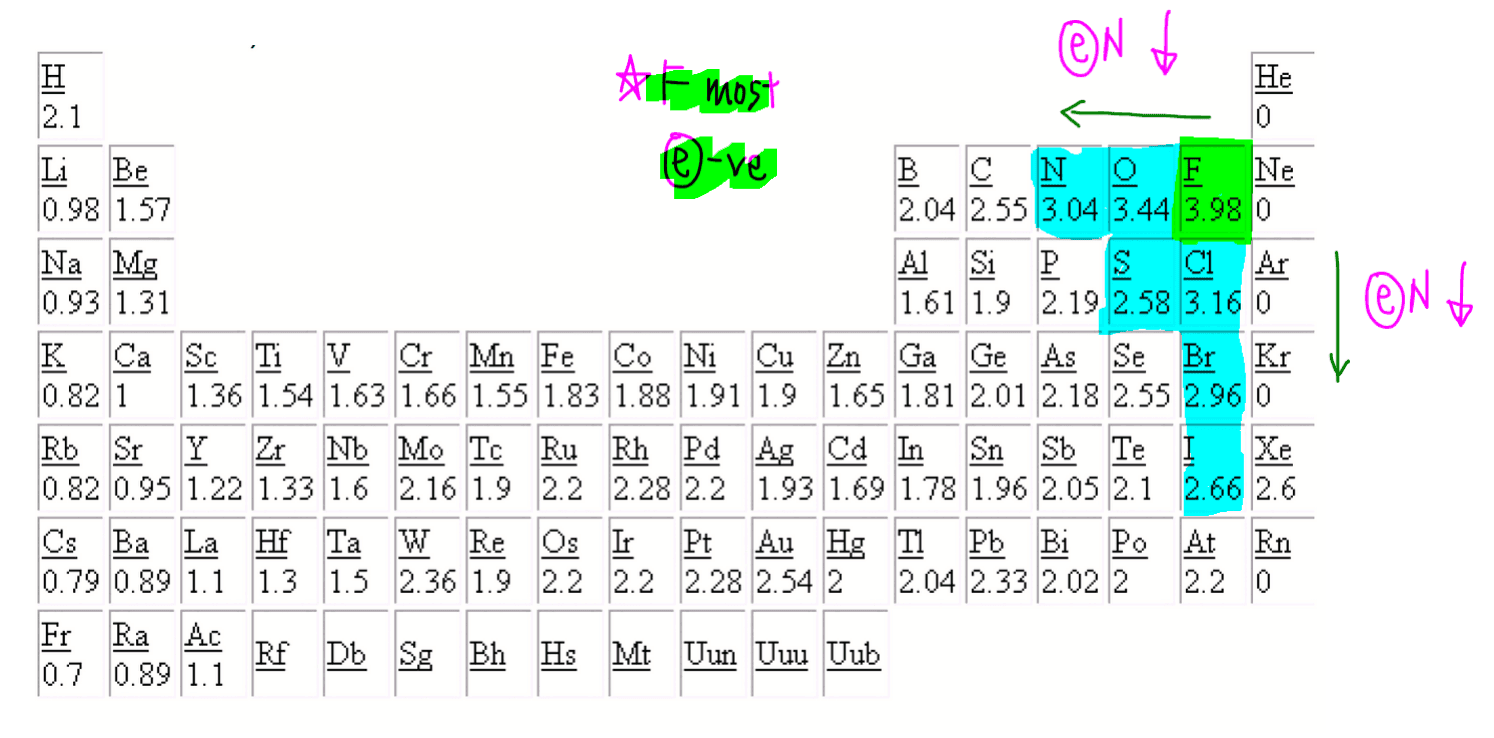
Hydroxide is an anion (negatively charged ion) made up of one oxygen atom to one hydrogen atom. Since oxygen is highly electronegative it is a common oxidizing agent. The oxidation number for O is 2- in most compounds. In this video, 2- is written above O. Another way to think about it is 1- being written above O2.
Oxidation Numbers Periodic Table Elements
This color periodic table contains the number, symbol, name, atomic mass and oxidation states of each element. The most common oxidation states are in bold text and predicted or unconfirmed states are in italics. This table is available for download as a PDF file and printed for offline use. For best printing, choose Landscape and 'Fit' for.
What is Oxidation State?
The oxidation number of a monatomic ion (by itself or as part of an ionic compound) is equal to its charge. Alkali metals - elements in the first column of the periodic table - will always have an oxidation number of +1; Alkali metals (column 2) are almost always +2
Printable Periodic Table With Oxidation Numbers Printable Word Searches
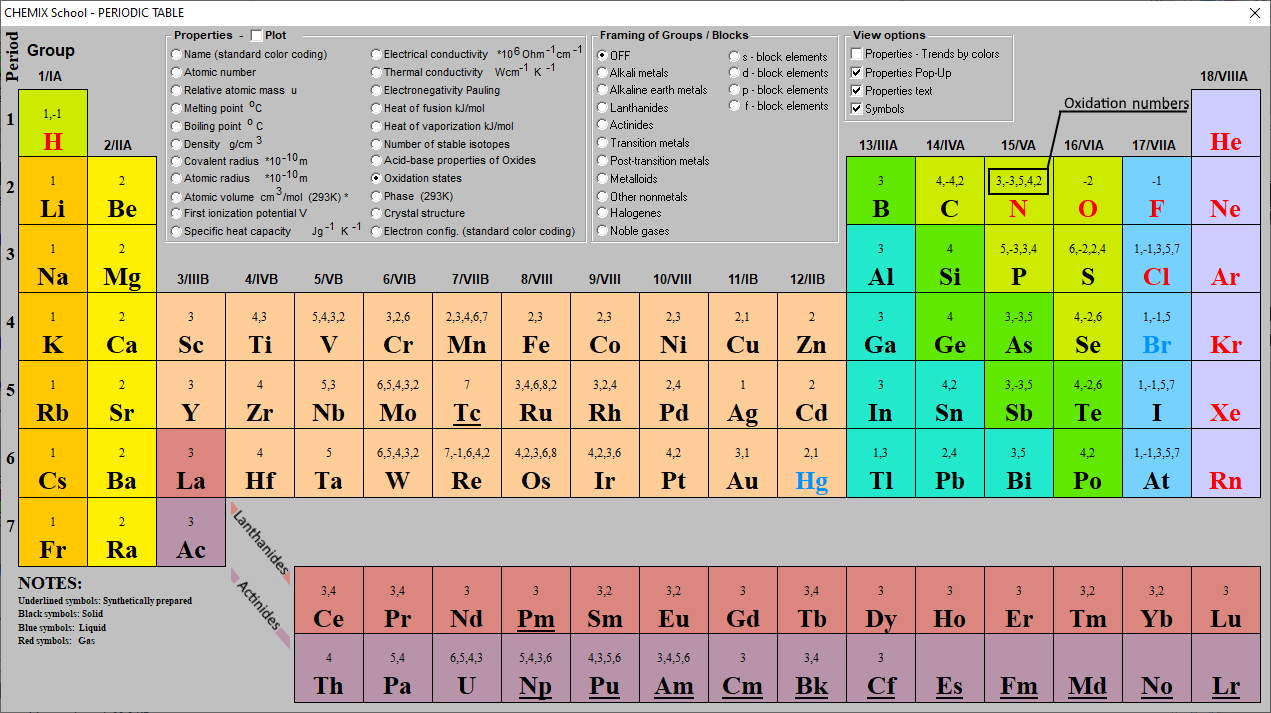
Interactive periodic table showing names, electrons, and oxidation states. Visualize trends, 3D orbitals, isotopes, and mix compounds.. 3D orbitals, isotopes, and mix compounds. Fully descriptive writeups. Periodic Table of Elements. Properties Electrons Isotopes Compounds. or buying a poster or wallet card, order number. 1H Hydrogen 1..
:max_bytes(150000):strip_icc()/PeriodicTableOxidation-BW-56a12da83df78cf772682bfe.png)
Periodic Table of the Elements Oxidation Numbers
However, most metals are capable of multiple oxidation states. For example, iron common has an oxidation number of +2 or +3. Halogens, on the other hand, have an oxidation state of -1. This periodic table contains the oxidation numbers of the elements as well as element numbers, symbols, names, and atomic weights.
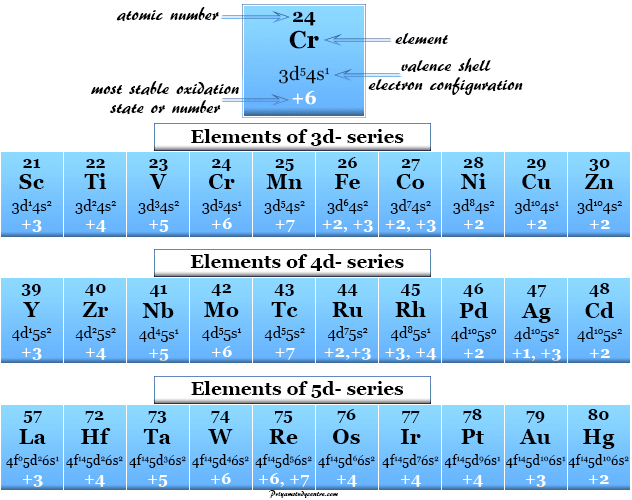
Ammonium On The Periodic Table
If oxygen is present, assume oxygen is -2. We created a video to explain how this is done. Rule 10 oxidation number examples: For example, in the nitrate ion NO 3-, the charge of the ion is -1. Then, using rule 2, we know the charge of the oxygens is 3 x-2 = -6. So nitrogen must have a charge of +5 to make the charge of the ion -1.

Downloadable Periodic Table Oxidation States
To calculate oxidation numbers of elements in the chemical compound, enter it's formula and click 'Calculate' (for example: Ca2+, HF2^-, Fe4 [Fe (CN)6]3, NH4NO3, so42-, ch3cooh, cuso4*5h2o ). The oxidation state of an atom is the charge of this atom after ionic approximation of its heteronuclear bonds. The oxidation number is synonymous with.

Free Printable Periodic Tables (PDF and PNG) Science Notes and Projects
Periodic Table with Oxidation Numbers Alkali Metal Alkaline Earth Transition Metal Basic Metal Metalloid Nonmetal Halogen Noble Gas Lanthanide H. 2 Oxidation Symbol . Numbers Name . Be Beryllium Si Silicon 4,2,3,6,8 3,4,6,8,2 4,2,3,6. Author: R. Musadya Created Date: 8/24/2021 3:55:34 PM.
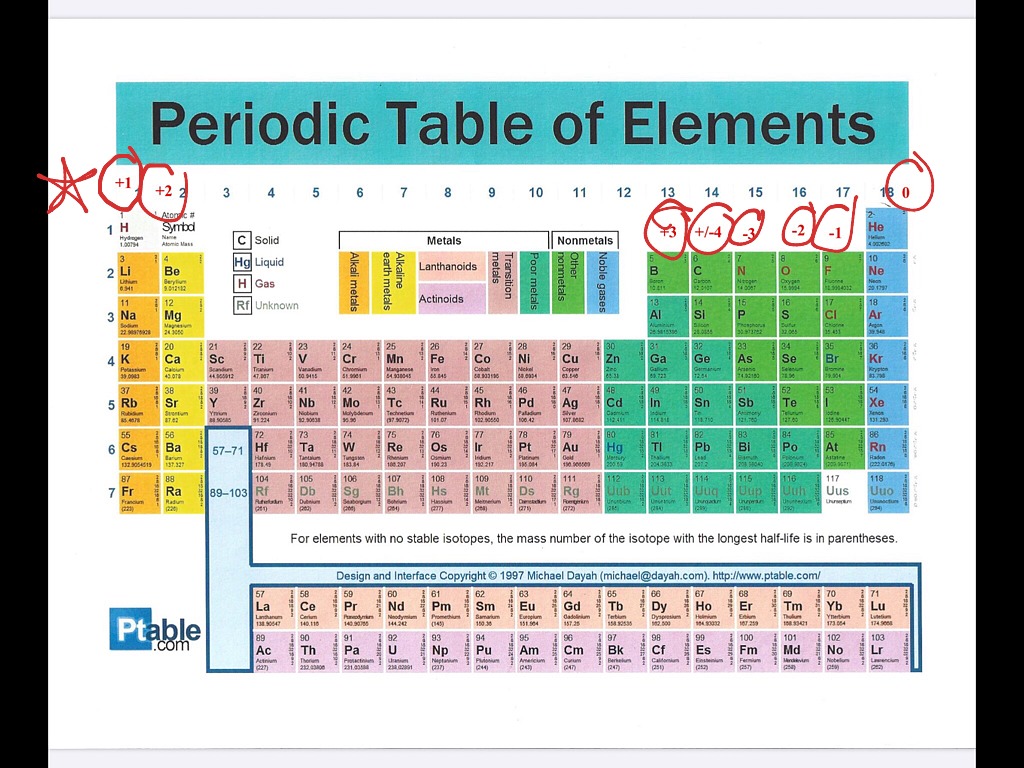
Oxidation explained Science, Chemicalreactions, Chemistry, Periodic
Color of the element symbol shows state of matter: black=solid: white=liquid: red=gas: grey=unknown
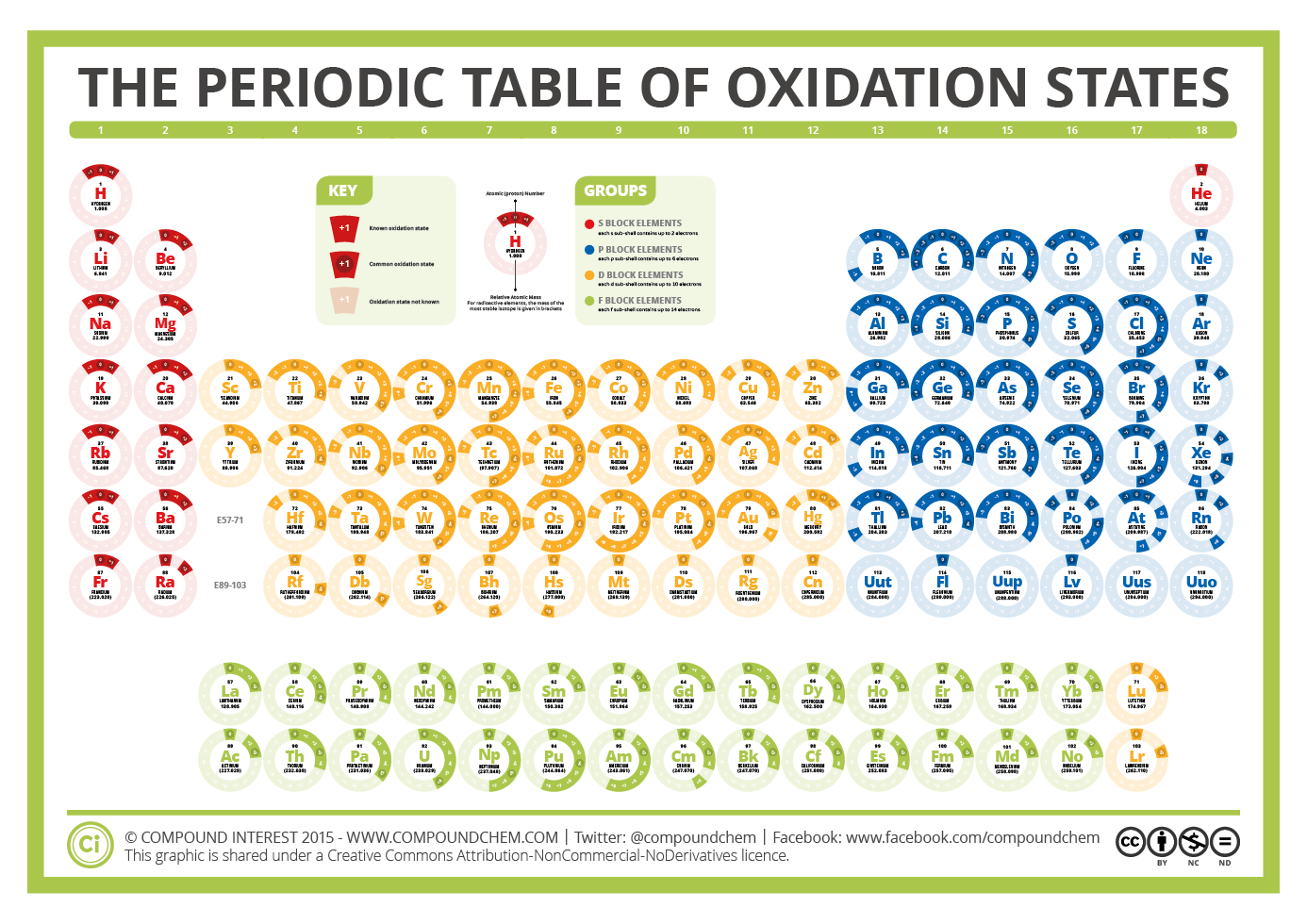
Compound Interest periodic table
These have an oxidation state of +1, the same as the charge on the ion. Similarly, iron (Fe) can lost two electrons to form the Fe 2+ ion, or lose three electrons to form the Fe 3+ ion. These have oxidation numbers of +2 & +3 respectively. With a chlorine ion (a chlorine atom that has gained one electron, Cl - ), the oxidation number would be.

Pin by Natalie Paes on chem Chemistry education, Chemical science
This number is defined as the formal charge on the atom if all bonds were assumed to be fully ionic. Knowing the oxidation state is very useful for example when balancing reduction-oxidation (redox) reactions. Figure 1: Oxidation states in the Periodic Table. Referred from: Article. Oxidation numbers for atoms, ions and compounds.

Oxidation Numbers Periodic Table Elements
Oxidation number of an atom when an element has combined with the same element. Table: oxidation numbers of according to the atomic number, first 20 elements. Oxidation states of s block. Hydrogen. Alkali Metals - Group 1. Alkali Earth Metals - Group 2. Oxidation states of p block elements. Group 3. Group 4.
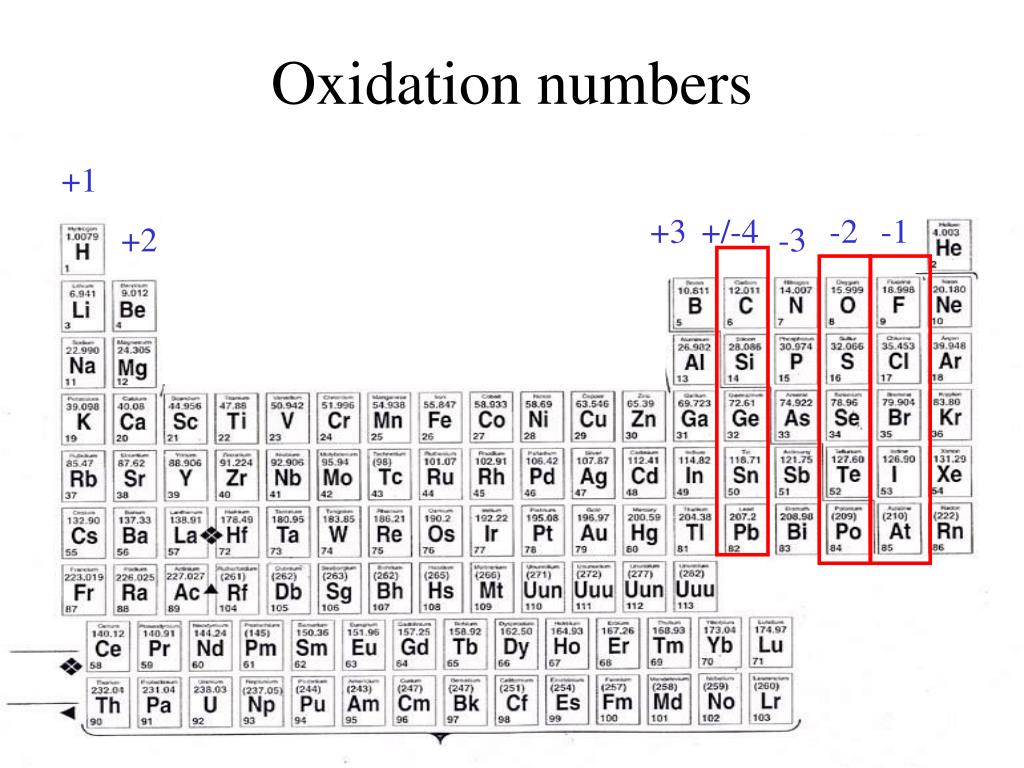
PPT Elements and Periodic Table PowerPoint Presentation, free
The positive oxidation state is the total number of electrons removed from the elemental state. It is possible to remove a fifth electron to form another the VO+2 VO 2 + ion with the vanadium in a +5 oxidation state. Each time the vanadium is oxidized (and loses another electron), its oxidation state increases by 1.
/PeriodicTableOxidation-BW-56a12da83df78cf772682bfe.png)
Periodic Table of the Elements Oxidation Numbers
The oxidation number of a monatomic ion is equal to the charge on the ion. For example, the oxidation number of chlorine in the Cl - ion is -1. The oxidation number of bromine in the Br - ion is -1. The oxidation number of sodium in the Na + ion is +1. Some atoms have several possible oxidation numbers. For example, iron can be Fe +2 or Fe +3.
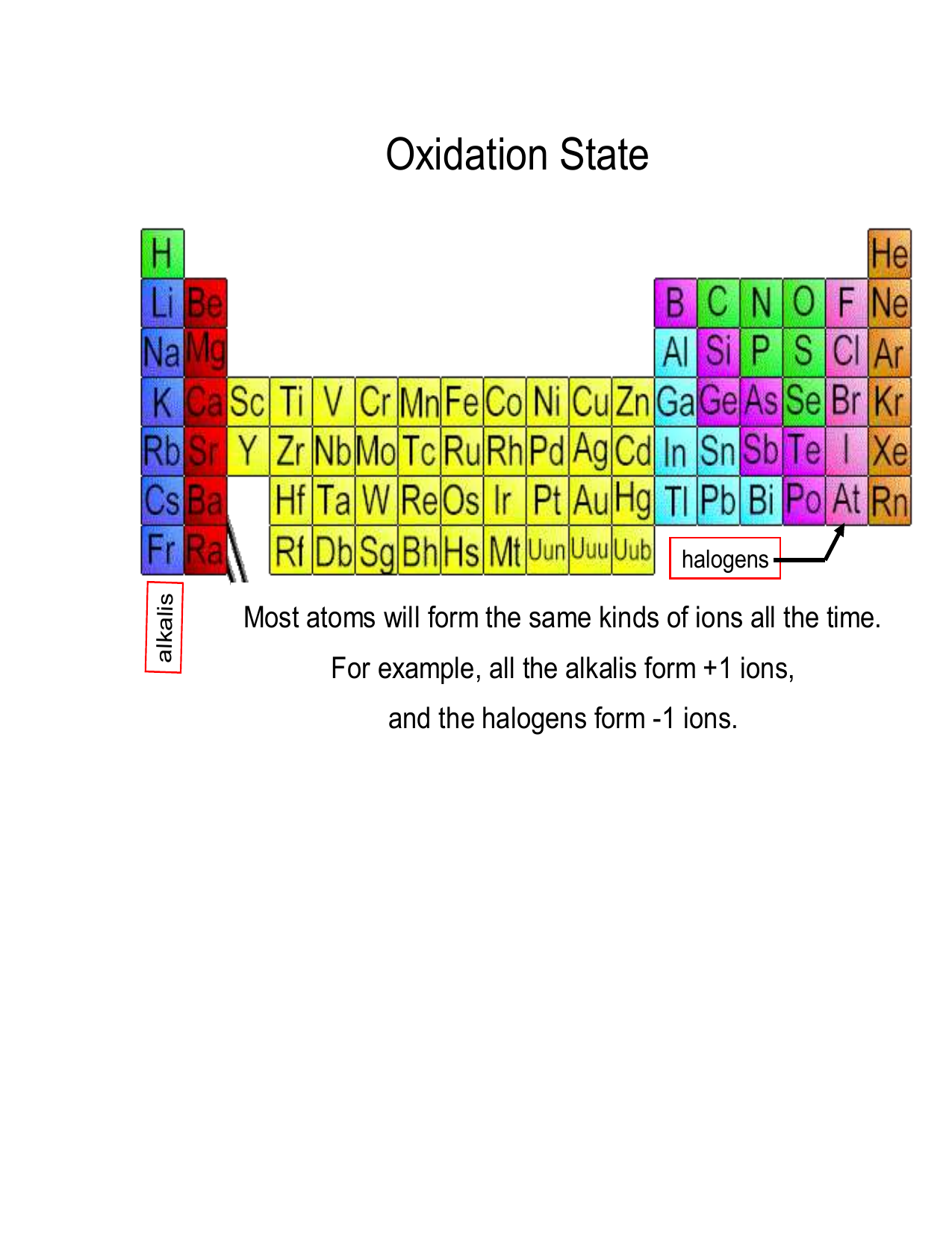
Oxidation Numbers
Oxidation numbers; Monoatomic ions oxidation numbers; Polyatomic ions oxidation numbers; Periodic table with oxidation numbers; Steps to writing chemical formulas; Molecular and Empirical Formulas; Molecular mass and formula mass; Types of chemical compounds; Binary compounds; Ternary compounds